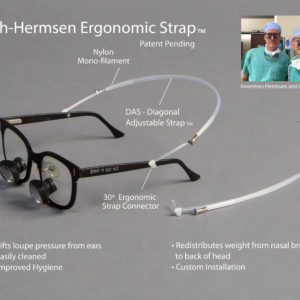
OMAHA, Neb. (January 22, 2018)—The University of Nebraska at Omaha and UNeMed, the technology transfer office at the University of Nebraska Medical Center, have signed a new services agreement for UNeMed to be the exclusive agent for UNO.
“We’ve worked with UNeMed in the past, and it always went well,” said UNO Associate Vice Chancellor Scott Snyder, PhD “We wanted to make that process easier and more efficient for everyone, and this arrangement does that.”
Dating back to the mid-1990’s, UNeMed has received about 50 new inventions from UNO inventors. Most UNO inventions, however, are more recent, emanating from UNO’s cutting edge Department of Biomechanics. Perhaps one of UNO’s more noteworthy innovations led to the creation of a new startup company, Avert, which promises to help athletic teams quickly and accurately diagnose concussions.
The new arrangement with UNeMed removes many institutional hurdles, allowing faculty, students and staff at UNO to work directly with the technology licensing experts at UNeMed.

Michael Dixon
“This is perfect for us,” UNeMed president and CEO Michael Dixon said. “We have a long history of working with UNO, and many UNMC researchers have strong collaborative ties to UNO. Hopefully, this will allow us to partner more with UNO researchers and help develop their innovations into products that can have a positive impact in the market.”
Common at virtually every major university in the nation, technology transfer offices work to protect the discoveries, innovations and inventions of their researchers. Tech transfer offices like UNeMed help identify industrial partners that can bridge the funding gap between a discovery and a product on store shelves.
“To have the expertise and experience of the people at UNeMed at our disposal is a huge asset,” Snyder said. “This should be a huge boost for many of our faculty, students and staff that want to see their ideas developed into products that help people.”
Initially established in 1991 as the tech transfer office for the University of Nebraska Medical Center, UNeMed now serves all University faculty, students and staff at both Omaha campuses and the College of Dentistry in Lincoln. UNeMed’s sister office, NUtech Ventures, serves the remainder of the University’s resources in Lincoln and Kearney.
Working primarily with academic researchers and inventors, UNeMed protects University discoveries and innovations, securing 489 patents over the last quarter century. With 106 years of combined technology development and commercialization experience, UNeMed staffers help propel those innovations through development into products that help improve the lives of people everywhere.

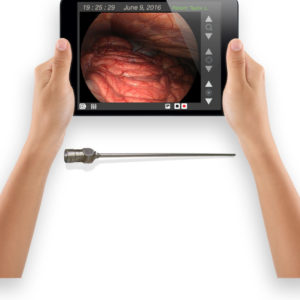
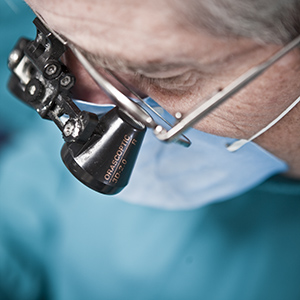
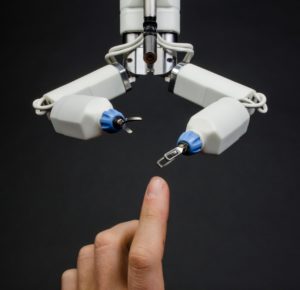
Virtual Incision’s robots are on the verge of transforming complicated, highly invasive open surgeries into relatively simple laparoscopic procedures.
Common routes to commercialization include establishing relationships with industrial partners for sponsored research agreements, signing licensing agreements with established commercial entities or building new startup companies. For example, a UNMC surgeon and a UNL engineer collaborated on a surgical robotic device to create Virtual Incision, a biomedical startup. Virtual Incision recently announced a successful Series B financing round of $18 million.
Virtual Incision is just one of 58 new startups UNeMed has help build in its 26-year history. UNeMed has also helped UNMC secure an additional $6.32 million in sponsored research while processing more than 1,330 new inventions from University faculty, staff and students.
Those 1,330 inventions eventually resulted in 230 licensing agreements over the years, which amounts to a track record that helped place the University of Nebraska 35th out of 230 in a recent report from the Milken Institute, a non-partisan think tank. The placement puts UNeMed, NUtech Ventures and the University of Nebraska in the top 15th percentile.
The 2017 report ranked the University of Nebraska’s tech transfer efforts ahead of such institutions as the Mayo Foundation (36), the University of Wisconsin (39), Ohio State University (55), and the University of Iowa Research Foundation (59), to name a few.
Read article
 OMAHA, Neb. (May 15, 2018)—Tyler Scherr, PhD, has been promoted from intern to a full-time licensing associate position with UNeMed, the technology transfer and commercialization office at the University of Nebraska Medical Center and the University of Nebraska at Omaha.
OMAHA, Neb. (May 15, 2018)—Tyler Scherr, PhD, has been promoted from intern to a full-time licensing associate position with UNeMed, the technology transfer and commercialization office at the University of Nebraska Medical Center and the University of Nebraska at Omaha.

















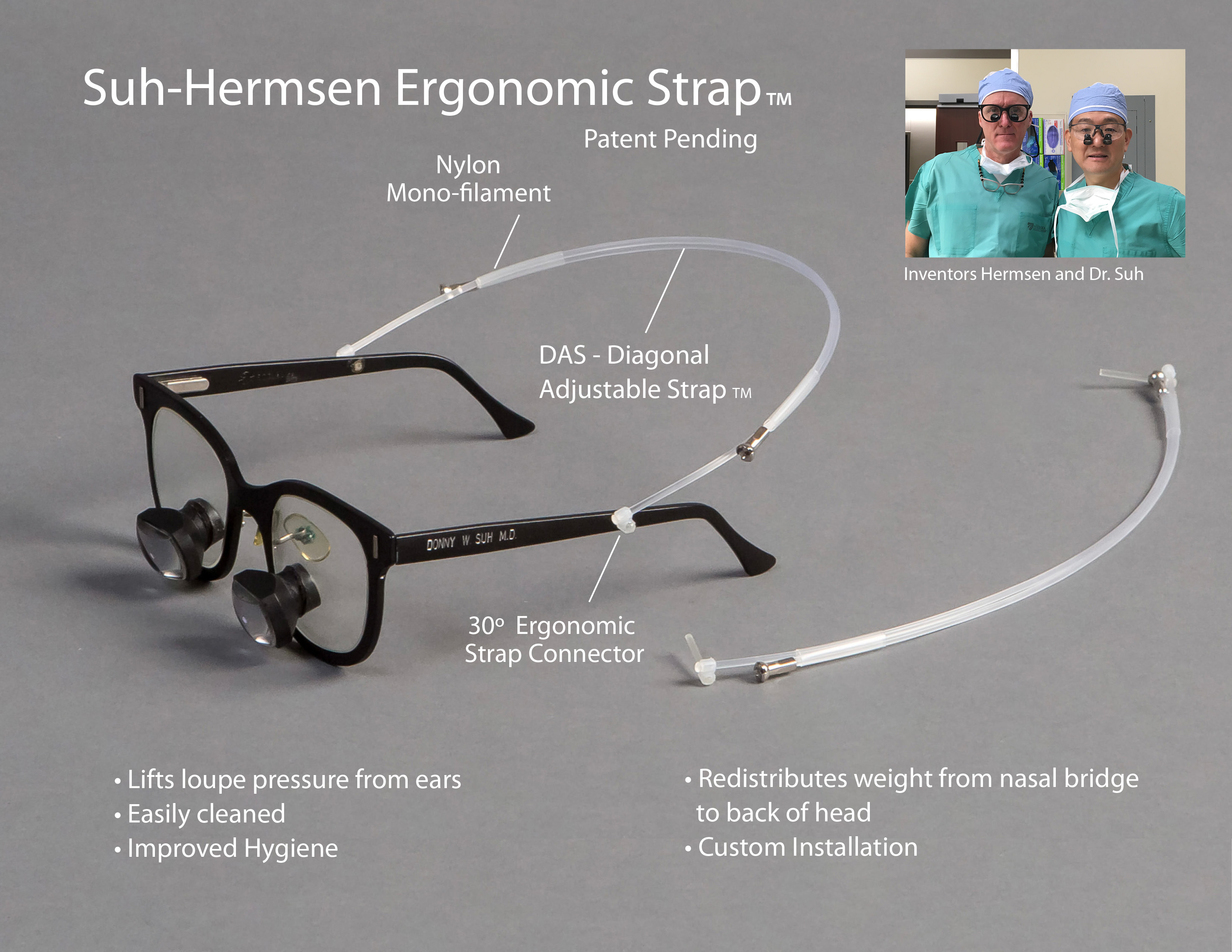












 Kansas City, Missouri (Jan. 25, 2018)—Nebraska entrepreneurs were the toast of the show at a regional entrepreneurial awards ceremony last week, receiving recognition for entrepreneurial leadership, best pitch, growth and the Innovator of the Year.
Kansas City, Missouri (Jan. 25, 2018)—Nebraska entrepreneurs were the toast of the show at a regional entrepreneurial awards ceremony last week, receiving recognition for entrepreneurial leadership, best pitch, growth and the Innovator of the Year.



 Launched in late 2016,
Launched in late 2016,  The addition of licensing associate Catherine Murari-Kanti, PhD, provided an unmistakable boost to UNeMed.com traffic. Some of the year’s most popular posts revolved around her, beginning with the announcement of her
The addition of licensing associate Catherine Murari-Kanti, PhD, provided an unmistakable boost to UNeMed.com traffic. Some of the year’s most popular posts revolved around her, beginning with the announcement of her 
 OMAHA, Neb. (December 15, 2017)—Virtual Incision Corporation, a startup company born from a collaborative research project at the University of Nebraska, completed an $18 million round of fund-raising, officials announced Tuesday.
OMAHA, Neb. (December 15, 2017)—Virtual Incision Corporation, a startup company born from a collaborative research project at the University of Nebraska, completed an $18 million round of fund-raising, officials announced Tuesday.
 OMAHA, Neb. (December 11, 2017)—William Payne, a doctoral student of pharmaceutical sciences at UNMC, was recently selected as the University of Nebraska’s top choice to present his business plan at the largest entrepreneurial conference in the region next month.
OMAHA, Neb. (December 11, 2017)—William Payne, a doctoral student of pharmaceutical sciences at UNMC, was recently selected as the University of Nebraska’s top choice to present his business plan at the largest entrepreneurial conference in the region next month.
 For example, if you are an investigator or researcher, you may want to share a new research discovery with a friend who works in the industry. You think that industry input is important in pivoting your research in the right direction. But, if you share your research without first having a CDA in place, you jeopardize your research and its commercialization potential. Filing a CDA protects your work and allows you to share unpublished information with academic collaborators or commercial partners.
For example, if you are an investigator or researcher, you may want to share a new research discovery with a friend who works in the industry. You think that industry input is important in pivoting your research in the right direction. But, if you share your research without first having a CDA in place, you jeopardize your research and its commercialization potential. Filing a CDA protects your work and allows you to share unpublished information with academic collaborators or commercial partners.