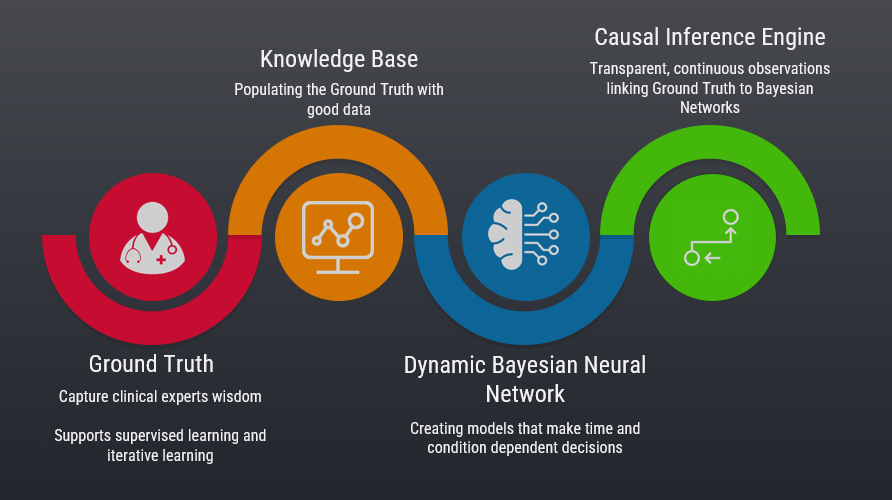
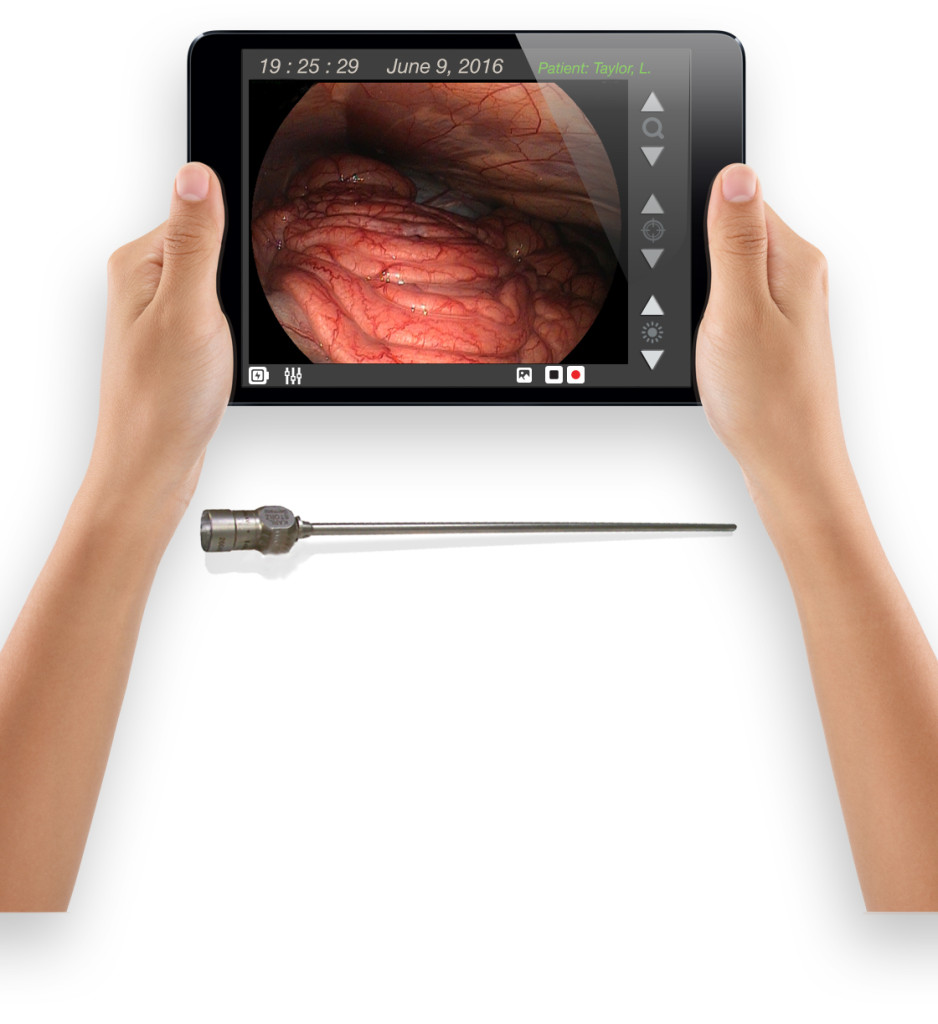
Sachin Pawaskar (left) and Thang Nguyen chat shortly before UNO’s IS&T Capstone presentation on June 1, 2018. The project was the realization of WeChart, Nguyen’s invention of a more efficient way to teach nursing students better clinical charting skills.
by Charlie Litton, UNeMed | June 18, 2018
Nourish an invention with a robust diet of diverse thoughts and competing ideas. Go ahead.
Maybe that sounds dangerous. But diversity of thought is a super-food for those delicate innovations as they embark on what will be an absolutely brutal journey.
It’s some tough love, to be sure, but do that and remarkable things can happen.
Maybe you would rather swaddle an idea in bubble wrap and wait for the phone to ring? There’s a word for that. It’s called neglect. All you’ll likely do then is watch that idea grow old, wither and die.
It happens. It doesn’t even have to be anyone’s fault, either. It’s just the dark side of innovation that happens everywhere. Sometimes there just isn’t enough oxygen to go around.
An ER nurse, Thang Nguyen, saw it happening to one of his ideas. As one of the more innovative people at the University of Nebraska Medical Center, most of his ideas come right out of the clinic. He has a long list of better, more efficient ways to do things like washing debris from wounds and helping patients breathe.
Just like any prolific inventor, Thang finds that sometimes those ideas work and sometimes they don’t. And, like too many clever ideas and better ways, sometimes they never get a real chance to fail or succeed.
One of Nguyen’s ideas—a software program to help nursing students learn the arcane art of charting patients in real time—was heading down a path toward obscurity.
Tapping new resources
Thang’s idea: A new way to train student nurses without risking patient safety or privacy. Medical schools don’t often have a lot of resources that involve advanced coding and application design.
Except, it turns out, they actually did: Right down the road at UNO’s Peter Kiewit Institute, a seven-minute drive with bad traffic. PKI is home to the University of Nebraska Omaha’s College of Information Science and Technology and the University of Nebraska-Lincoln’s College of Engineering.
“There’s a lot of talent here at PKI that no one at UNMC even knows about,” Nguyen said.
Creating that well-balanced meal of innovation super food is not that hard. It turns out, the first, most important step is just getting the right people in the same room. What Nguyen really needed wasn’t another person in healthcare. What he needed was someone completely removed from healthcare. He needed a software engineer.
They have those in spades at UNO’s College of Information and Science Technology, or IS&T for short. They also make some serious hay with a capstone program where IS&T grad students develop concepts and ideas into actual things. Working prototypes ready for full-scale testing and, in some cases, widespread use.
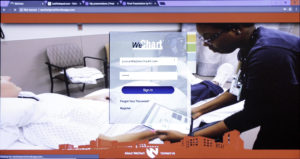 Nguyen’s idea, called WeChart, lay dormant for four years. Then it joined the capstone program.
Nguyen’s idea, called WeChart, lay dormant for four years. Then it joined the capstone program.
Working with UNO, Nguyen said he got more “accomplished in one semester than we did in the previous four years.”
It took a full academic year and two teams of students, but Nguyen now has a final product that, once upon a time, only lived in his imagination. It now lives in the real world.
And it will be used in the real world. UNMC’s emergency medicine department will train their student nurses with it. If all goes well, we might see other teaching hospitals and medical schools adopt the charting training program.
That’s great. Wonderful, in fact.
The bigger picture
For my money, the University of Nebraska—and other similar institutions, if they’re paying attention—should be excited about a more important prototype. I’m talking about the relationship forged between two disparate departments and two wildly different campuses.
“Medical people think one way,” Nguyen said, “a software engineer thinks one way…We should not forget that we are there to solve one central problem even if there are multiple ways of looking at it.”
For Nguyen, the “problem” of the hour was too-common charting errors from inexperienced and poorly trained young nurses. His WeChart application is one solution. We’ll find out real soon if that solution works like we all hope it does.
But there are bigger problems. Unknown problems. Problems the University of Nebraska can identify and solve, if the right people can get together.
“Why can’t we be a powerhouse,” asked the capstone program leader, Sachin Pawaskar, PhD, a research technology fellow at IS&T. “I think we have that potential.”

Sachin Pawaskar, leader of UNO’s IS&T Capstone program, addresses the gathering following his team’s WeChart presentation on June 1, 2018.
Pawaskar, who also holds an MBA, made it clear the WeChart project was critically important for the capstone program. He wanted a “showcase of what a successful partnership and relationship could be.”
That showcase will be on full display on campus at UNMC in the coming semesters as young nursing students put WeChart through the paces.
Nguyen’s idea will finally get the chance to sink or swim on its own merits, which is all anyone can really ask.
But more than that, UNMC and UNO now have a buffet line that can fuel some innovative ideas across a treacherous divide—the chasm between the sketch of a promising concept and a final product that actually makes life better.
“I really think that the healthcare area is just set up for an IT revolution,” Pawaskar said shortly after his students presented their WeChat project to Nguyen and dozens of others. Pawaskar added: “I really feel that healthcare can be totally revolutionized if we can incorporate IT at the right places at the right levels…It’s not any silver bullet, but there’s so many things you can do.”
Nguyen nodded: “And the best part? All the pieces are already here.”
Read article















 Nguyen’s idea, called WeChart, lay dormant for four years. Then it joined the capstone program.
Nguyen’s idea, called WeChart, lay dormant for four years. Then it joined the capstone program.