by Joe Runge, UNeMed | June 2, 2016
Your veins and arteries are not just pipes. They expand and contract. They grow and shrink. They repair themselves. Like every part of your body, they are made of living tissue that responds to what you do and the environment around you.
 Pipes can’t do that.
Pipes can’t do that.
Pipes aren’t lined with cells that sense rate, direction and flow. Pipes aren’t ringed with muscles that expand and contract in response to momentary needs. Pipes do not grow in order to increase flow to your shower. They do not shrink and wither away when you switch the water off.
The diseases that afflict your circulatory system are not a failure in plumbing. If blood vessels were mere pipes, then atherosclerosis would just be a clog. It isn’t. A doctor cannot simply snake out your coronary arteries like a plumber. The narrowing of your blood vessels is the result of a complex immunological reaction that occurs between your blood vessels and the chemicals in your blood. Doctors physically pry open your arteries in order to treat your coronary artery disease, but they almost immediately start narrowing again.
That narrowing is a reaction to what’s in your blood—the chemically destructive byproducts of fat and cholesterol, or free radicals. It is actually your body’s immune system’s reaction to the free radicals that drives atherosclerosis.
New research about atherosclerosis suggests that your immune system’s ability to clear free radicals from your arteries is a critical factor to assess your risk of heart attacks, it’s your immune state that helps predict if you die young from a heart attack. The way your immune system reacts to the fat and cholesterol in your blood may be as important as your diet in assessing your risk of a heart attack.
A new blood test invented by a team of scientists and doctors at the University of Nebraska Medical Center measures your immune response to how fat and cholesterol break down in your blood. The team was able to differentiate people who have stable atherosclerotic disease from those that had sudden heart attacks. For the first time, doctors can predict that sudden heart attack–well before it happens.
Such a ground-breaking concept requires a deep understanding of the cardiovascular system, and how it reacts to subtle changes. While artery disease can be a measure of the immune system response, blood vessels also react to subtle physical changes as well. Blood vessels do not just react to what’s in your blood, they also react to how your blood flows. Just as your blood vessels narrow due to atherosclerosis, they can also grow.
As a body builder pumps iron, his blood vessels grow. Cells inside his veins react to the increase in blood flow to feed his muscles. The same process that pops veins on bulging biceps will help some chronically ill people–patients in kidney failure.
Your kidneys filter the waste out of your blood. If they fail, you’ll have about a week or two before all that waste in your body rises to toxic levels high enough to kill you. You’ll need a new kidney or a machine to fill in until a suitable donor can be found. Filtering the blood through a machine, hemodialysis, saves countless lives every day. But it requires access to a large blood vessel.
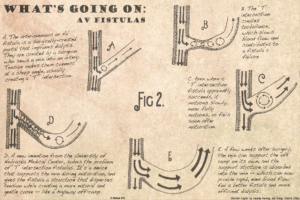 Doctors have to make that blood vessel by creating something called an arteriovenous fistula. The surgeon cuts a vein in the patient’s arm and sutures it to a nearby artery. The artery has much greater blood pressure and blood flow than the vein, so when the procedure is successful, the vein swells to accept the increased blood flow.
Doctors have to make that blood vessel by creating something called an arteriovenous fistula. The surgeon cuts a vein in the patient’s arm and sutures it to a nearby artery. The artery has much greater blood pressure and blood flow than the vein, so when the procedure is successful, the vein swells to accept the increased blood flow.
Most fistulas fail, usually when the vein clots after the surgery. Marius Florescu, M.D., believes the failure is due to the pattern of blood flow. He observed that most blood vessels connect at very gentle angles, like a highway off ramp. Blood vessels facilitate blood flow at highway speeds by making very gradual curves. Current research supports that blood vessels work best at highway speed. The body builder’s veins pop because the blood inside them is moving fast through the long, gently curving blood veins. His veins are growing in response to the blood’s laminar flow.
Fistulas are made at hard, right angles. Instead of a nice, long off-ramp it comes to a sudden stop at a T-intersection. That T-intersection is the opposite of laminar flow. Instead of highway speed it is stop-and-go traffic.
The cells lining the inside of the vein are able to detect that traffic, which makes the vein reluctant to grow. The vein is looking for highway speed and when it finds heavy traffic, the vein will shrink to protect the rest of the vascular system from whatever is disrupting blood flow.
Dr. Florescu invented a new device that creates on-ramps instead of T-intersections. The device is a platform that the surgeon can place inside the vein during fistula surgery. It holds the fistula at a gentle angle so that blood flows through the fistula in a less turbulent way. The platform is inexpensive and actually makes the surgical procedure faster, safer and more likely to succeed. After the connection between the artery and vein is solid enough, the device will disappear being resorbed by the body.
What your blood vessels do is invisible. They dilate and constrict in an instant response to your environment. They grow and shrink in response to your body’s gradual changes. They are ground zero for chronic, immunological changes in your body.
The only time we notice them is when they fail–a lot like the pipes in your home.
The new blood test to predict risk of heart attack and the fistula maturation platform take results from brilliant research and apply them to improve patients’ lives. The scientists and doctors have so much more to learn about the complex wonder that is the human body.
Perhaps the first barrier to the next discovery is to look at a blood vessel and see more than a pipe.
Read article
 OMAHA, Neb. (June 16, 2016)—UNeMed announced today a recently struck licensing deal with a diagnostics and medical device company in Memphis, Tenn., HealthChart LLC, to further develop a new test that could significantly improve treatment strategies for certain types of rare blood cancers—peripheral T-cell lymphoma.
OMAHA, Neb. (June 16, 2016)—UNeMed announced today a recently struck licensing deal with a diagnostics and medical device company in Memphis, Tenn., HealthChart LLC, to further develop a new test that could significantly improve treatment strategies for certain types of rare blood cancers—peripheral T-cell lymphoma.
 An invention begins with an idea. For example, say a researcher is studying the effects of second-hand smoke on the lungs of ferrets. The researcher needs a certain material to conduct the study. As a legal intern, I helped to draft and negotiate a
An invention begins with an idea. For example, say a researcher is studying the effects of second-hand smoke on the lungs of ferrets. The researcher needs a certain material to conduct the study. As a legal intern, I helped to draft and negotiate a  OMAHA, Neb. (June 6, 2016)—UNeMed expanded its approach to commercializing its portfolio of inventions and innovations, announcing today a deeper relationship with Straight Shot, an Omaha accelerator focused on helping technology startups.
OMAHA, Neb. (June 6, 2016)—UNeMed expanded its approach to commercializing its portfolio of inventions and innovations, announcing today a deeper relationship with Straight Shot, an Omaha accelerator focused on helping technology startups.


 OMAHA, Neb. (May 17, 2016)—There is $2.5 billion in federal grant money available through the SBIR/STTR program, and an upcoming “road show” will help researchers, innovators and entrepreneurs learn how they might make use of the funding opportunities.
OMAHA, Neb. (May 17, 2016)—There is $2.5 billion in federal grant money available through the SBIR/STTR program, and an upcoming “road show” will help researchers, innovators and entrepreneurs learn how they might make use of the funding opportunities.


 Yes. Anyone can be an inventor.
Yes. Anyone can be an inventor.




 OMAHA, Neb. (April 12, 2016)—Modern innovation and prototype development will play center-stage during a series of public events at the University of Nebraska Medical Center when it hosts the 3D Printing Invent-a-Thon as part of the
OMAHA, Neb. (April 12, 2016)—Modern innovation and prototype development will play center-stage during a series of public events at the University of Nebraska Medical Center when it hosts the 3D Printing Invent-a-Thon as part of the 


 You don’t have to be Mark Zuckerberg. Entrepreneurs are not just misunderstood geniuses that leave college early to intensely build their big ideas. They are also professionals that have solved problems for their employers and launched new products. They are employees that have accumulated valuable skills and now want to put those skills to work for themselves.
You don’t have to be Mark Zuckerberg. Entrepreneurs are not just misunderstood geniuses that leave college early to intensely build their big ideas. They are also professionals that have solved problems for their employers and launched new products. They are employees that have accumulated valuable skills and now want to put those skills to work for themselves.