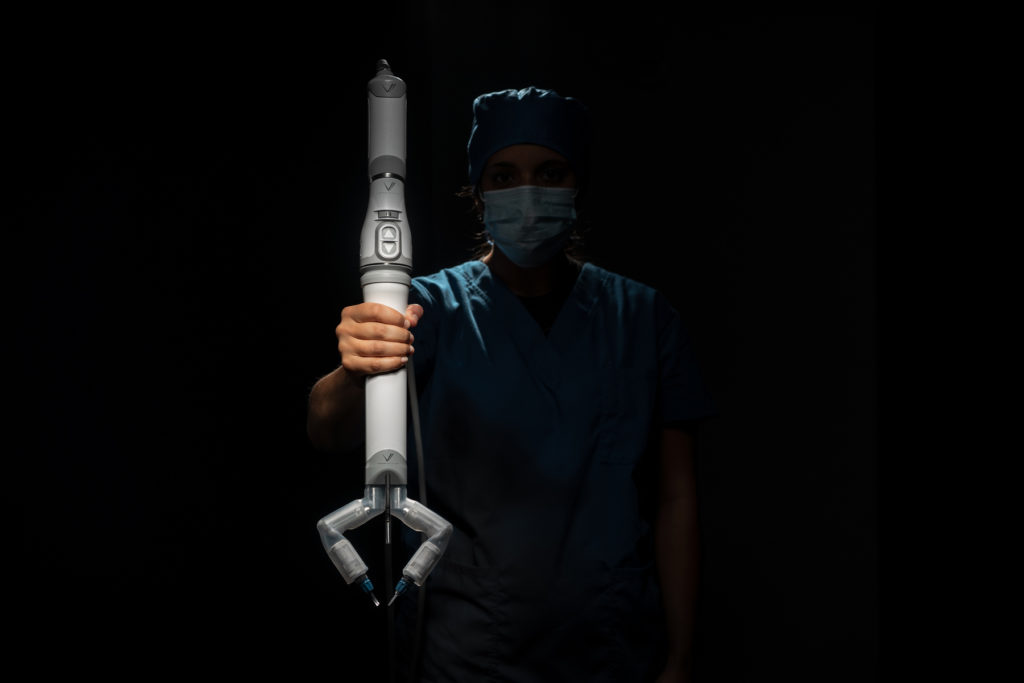
LINCOLN, Nebraska (February 24, 2024) — The FDA has granted Virtual Incision approval to use its ground-breaking surgical robotics platform, called MIRA, for adult patients undergoing colectomy procedures. FDA approval finally opens the door for the Nebraska-born innovation to be used in hospitals, possibly enabling wider access to minimally invasive procedures to millions of Americans.
“While this is an important milestone, there’s always more to do,” said Michael Dixon, PhD, president and CEO of UNeMed, the tech transfer and commercialization office at UNMC that helped establish Virtual Incision. “The surgical robots need to be made and surgeons need to be trained to use them. But for patient safety and functionality, it’s passed the major hurdles. It’s been a long, decade-plus odyssey to go from an idea to an approved product, so this didn’t happen overnight. And getting to widespread adoption probably won’t happen overnight either; however, I am optimistic that this technology will have a major impact on healthcare over the next decade.”
MIRA, short for Miniature In vivo Robotic Assistant, is the product of a cross-campus collaboration between University of Nebraska-Lincoln robotics professor, Shane Farritor, PhD, and former UNMC surgeon Dmitry Oleynikov, MD.
It will be initially limited to colectomies, also referred to as a colon resection. Considered a major surgery, colon resections are among treatment options for patients with lower gastrointestinal diseases including diverticulitis, colon lesions and inflammatory bowel disease. Colon resections often involve a large incision so the surgeon may remove the damaged or diseased portion of the bowel.
It may take months to recover fully from such an open procedure, but recovery would be significantly reduced when the same procedure is performed laparoscopically. A surgical robot like MIRA, can provide that option to more patients.
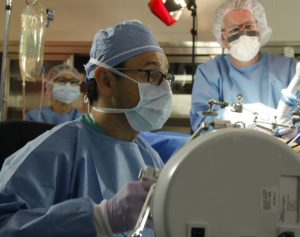
Former UNMC surgeon Dmitry Oleynikov (left) and UNL engineer Shane Farritor (right) test an early prototype of its surgical robot several years ago in Omaha. Their collaboration created a startup company, Virtual Incision, which now has FDA clearance to use their robotics technology in human adults.
Other surgical robotic options exist, but they are massive, main-frame units that take up an entire room and reach into the body from outside the patient. MIRA, however, is a small, self-contained surgical device that is inserted through a single midline umbilical incision in the patient’s abdomen. It does not require a dedicated operating room or specialized infrastructure, so it is expected to be significantly less expensive than existing robotic alternatives for laparoscopic surgery. Virtual Incision’s technology promises to enable a minimally invasive approach to surgeries performed today with a large open incision.
Virtual Incision said it will begin commercializing MIRA in select centers across the United States, and will eventually ramp up to additional sites over time. Virtual Incision added that it will seek additional approval for uses in other conditions related to gynecology, general surgery, urology and other soft tissue and solid organ surgery.
Studies of MIRA in gynecological procedures are already planned for later this year; and a new version of MIRA that is designed for general surgery is expected to be used in a first-in-human study outside the U.S., also later this year.