Description
Directly edit the genes of zygotes with ‘improved-Genome editing via Oviductal Nucleic Acids Delivery’
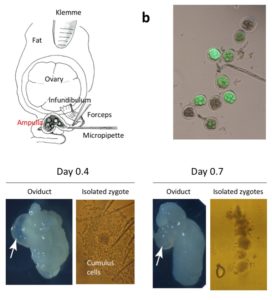
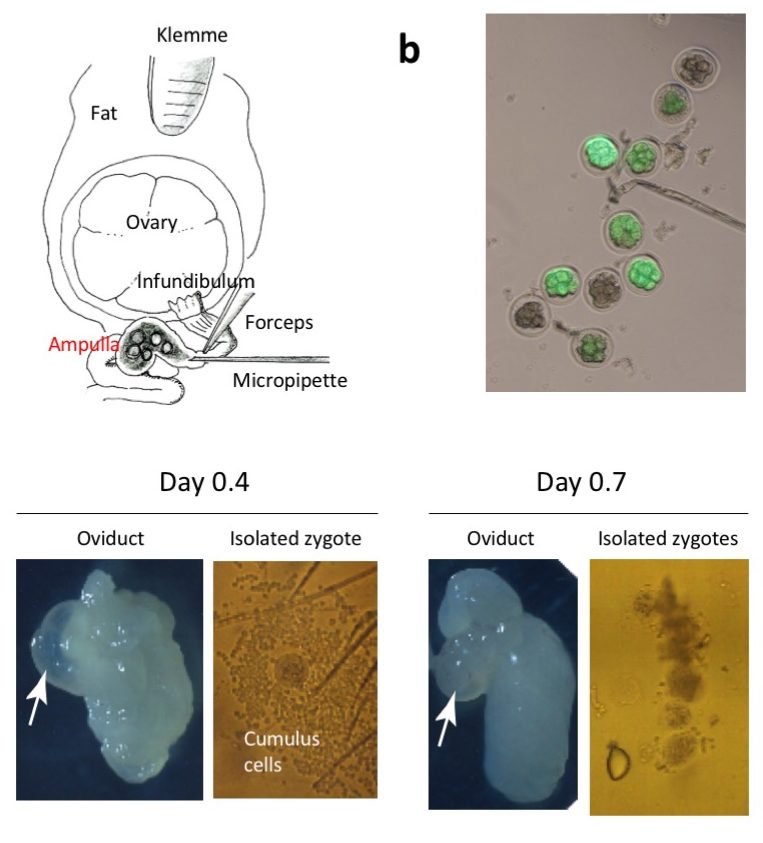
Diagrammatic illustration showing the anatomical structures of ovary and oviduct and
the surgical equipment used for GONAD procedure. A small amount of solution is injected by direct insertion of a glass micropipette through oviduct wall located at the region between the ampulla and infundibulum. Immediately after injection, in vivo electroporation is performed on the entire oviduct.
The oviduct dissected on day 0.7 exhibits shrinkage of the ampulla (arrow), and zygotes isolated from the day 0.7 ampulla have fewer cumulus cells, which will less likely hamper the uptake of exogenous nucleic acids/proteins upon electroporation
The University of Nebraska Medical Center’s Channabasavaiah Gurumurthy, PhD, collaborated with Japanese researchers Masato Ohtsuka, PhD, and Hiromi Miura, PhD, of Tokai University’s School of Medicine, invented i-GONAD. Traditionally, generating transgenic mouse-models involves three critical steps: isolation of zygotes from sacrificed females, zygote micromanipulation ex vivo and transfer of these modified zygotes into another set of female mice. This process has remained unchanged for over four decades and is laborious – requiring a high level of expertise, expensive and time-consuming.
The i-GONAD technique relives these steps by delivering genome editing nucleic acids and CRISPR components into embryos in situ. The process involves the exposure of the ovaries and oviduct of pregnant mice bearing E0.7 embryos. The genome editing reagents are injected into the oviductal lumen and the entire oviduct is subjected to electroporation using tweezer-type electrodes. These in situ, genome edited embryos are allowed to develop to term and genotyped for the targeted mutation.
This technique, when used in combination with Easi-CRISPR, another invention from Dr. Gurumurthy and Dr. Ohtsuka, inserts long single stranded DNA donor format with insertion efficiency as high as 100 percent. This combination changes the landscape of transgenic animal-model generation and provides a tool that is easy-to-perform and has high efficiency.
To discuss licensing opportunities about this technology, contact Amanda Hawley, PhD, at ahawley@unmc.edu or 402-310-5602.